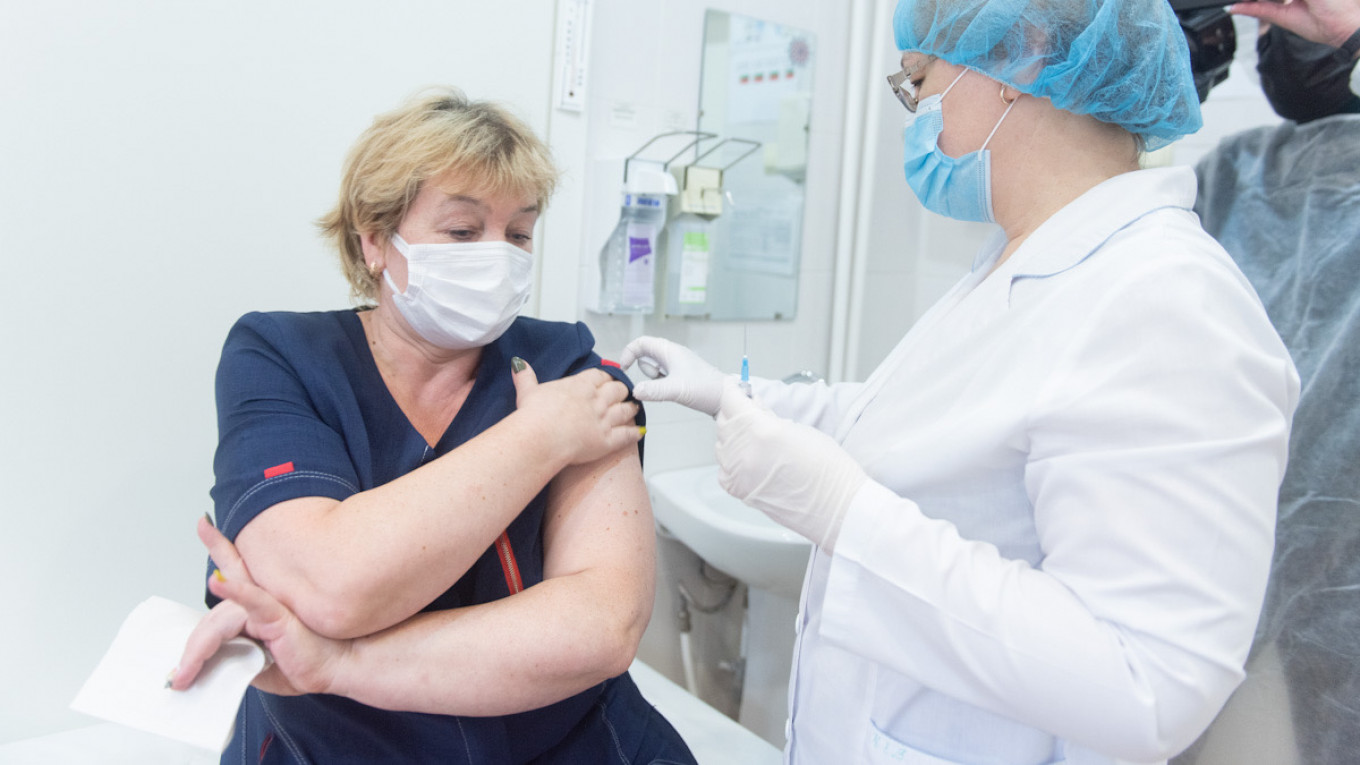
Russia’s coronavirus vaccine faces equipment shortages that could delay it from entering mass production, President Vladimir Putin said Thursday as reports suggested that developers have paused clinical trials due to the shortages.
Staff at eight of the 25 trial clinics in Moscow said that they temporarily halted the vaccination of new volunteers with Sputnik V, Reuters reported Thursday, citing some of them as saying that they had used up the allocated doses. An unnamed representative of the contract research organization helping oversee the two-shot vaccine's trials, Crocus Medical, told the news agency it expects vaccinations to restart by around Nov. 10.
“The only question now is to ensure the needed volume of industrial production,” Putin said at an investment forum. “There are certain problems linked to the availability or lack of the necessary equipment, or ‘hardware’ as they say, for mass production.”
A Crocus Medical representative linked the halt in inoculations to “colossal demand for the vaccine and they are not producing enough to keep up,” according to Reuters.
Sputnik V’s developer, the state-run Gamaleya Research Institute of Epidemiology and Microbiology, reportedly said it slowed vaccinations of new volunteers to focus on the second components, which are injected 21 days after the first.
The heads of Gamaleya and Crocus Medical denied that the trial had been paused.
“The trial continues, and there are enough supplies of vaccine,” Reuters quoted Crocus Medical director Alexei Butylin as saying.
“Everything is on track. It’s simply that the gap between [the number of people inoculated with] the first and second dose is quite significant,” Gamaleya director Alexander Gintsburg was quoted as saying.
Moscow’s deputy mayor said Friday that 10,000 volunteers have received both components so far, adding that none of them experienced “serious” side effects.
The double-blind trial for Sputnik V’s long-term safety and effectiveness involves 40,000 volunteers, 10,000 of whom are expected to receive a placebo.
Gintsburg noted this week that some of the volunteers have contracted Covid-19 since receiving the vaccine. He suggested that they may have received a placebo during the trial, saying that it would be possible to find out in mid-November before the trials wrap up.
A Message from The Moscow Times:
Dear readers,
We are facing unprecedented challenges. Russia's Prosecutor General's Office has designated The Moscow Times as an "undesirable" organization, criminalizing our work and putting our staff at risk of prosecution. This follows our earlier unjust labeling as a "foreign agent."
These actions are direct attempts to silence independent journalism in Russia. The authorities claim our work "discredits the decisions of the Russian leadership." We see things differently: we strive to provide accurate, unbiased reporting on Russia.
We, the journalists of The Moscow Times, refuse to be silenced. But to continue our work, we need your help.
Your support, no matter how small, makes a world of difference. If you can, please support us monthly starting from just $2. It's quick to set up, and every contribution makes a significant impact.
By supporting The Moscow Times, you're defending open, independent journalism in the face of repression. Thank you for standing with us.
Remind me later.