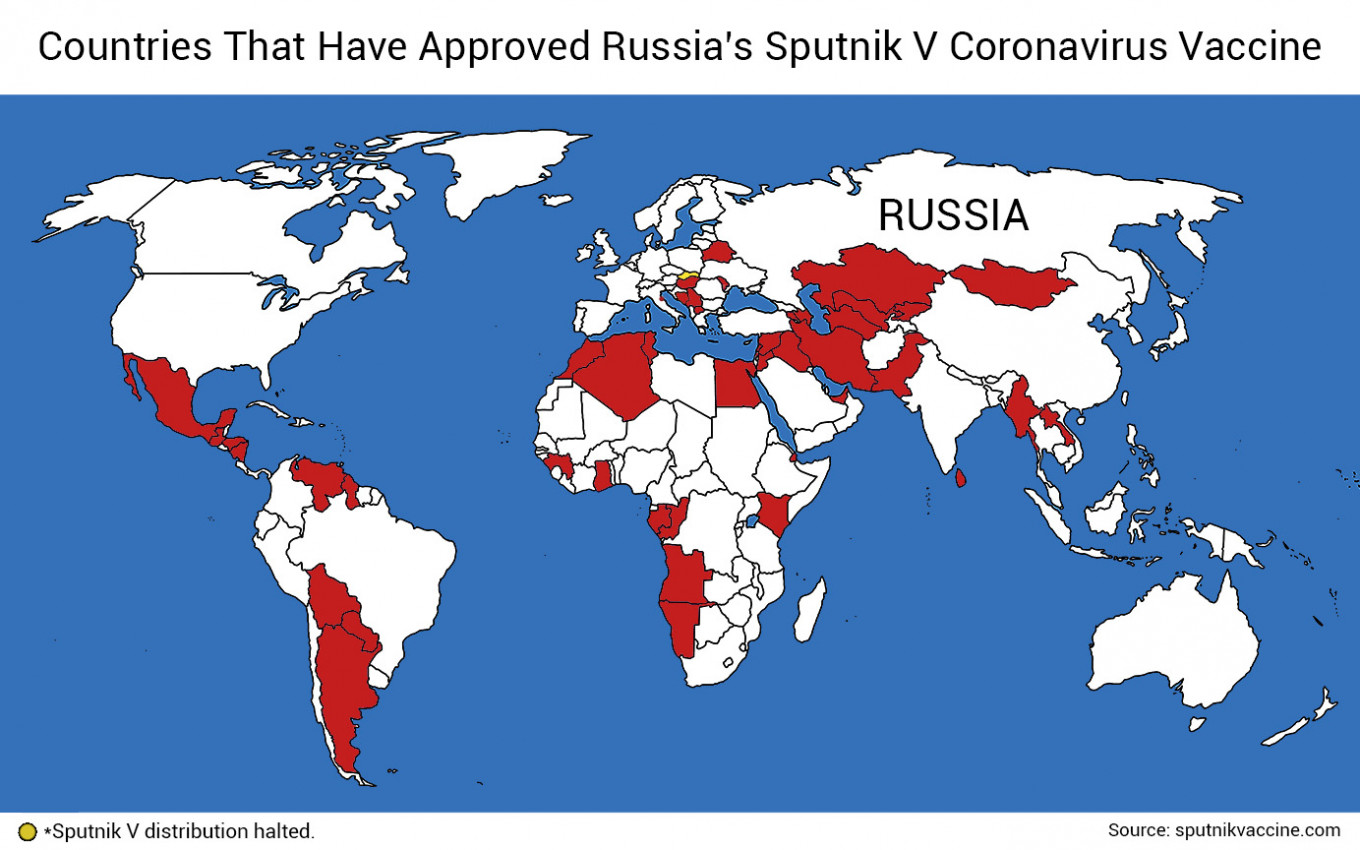
While Russia’s Sputnik V coronavirus vaccine has already been authorized by over 50 countries worldwide, it has yet to make its way into one of the world’s largest markets, the European Union.
On March 8, an official at the EU's medicines regulator was quick to dismiss the idea of granting emergency authorization for Russia's vaccine, comparing it to "Russian roulette." Just one week later, the tides seem to have turned in Russia’s favor: Sputnik V's developers have announced production agreements in key EU countries, while unconfirmed reports have indicated that vaccine-purchase negotiations between Russia and the EU are on the horizon.
Here is a look at what has happened on the European front of Russia’s vaccine diplomacy so far and what it means for both parties:
Has Russia’s vaccine been approved in the EU?
Sputnik V is not yet authorized for use in the European Union.
The EU’s medicines regulator, the European Medicines Agency (EMA), launched the first step of the approval process, known as “rolling review,” earlier this month. During this process, the regulator will analyze existing published data on the vaccine’s safety and effectiveness and decide if there is enough information for Russia to apply for authorization.
The EMA has not specified how long the process might take, though in the cases of other vaccines already approved for use in the EU — the Pfizer, AstraZeneca and Moderna jabs — it took 2-3 months from the start of the review.
Can individual EU member states use the vaccine?
EU members can approve vaccines for emergency use within their borders, though EMA management board chair Christa Wirthumer-Hoche has urged members to refrain from doing so ahead of the EMA’s decision.
So far, Hungary is the only EU member to have granted emergency national authorization to Sputnik V and is already using the jab in its mass vaccination drive. Slovakia’s health ministry also issued special approval for the use of Russia’s vaccine, but its distribution within the country was halted amid political controversy.
What are the vaccine production agreements?
Swiss pharmaceutical company Adienne Pharma & Biotech SA became the first Europe-based company to conclude a deal with Sputnik V’s marketer and funder, the state-run Russian Direct Investment Fund (RDIF), to produce the vaccine at its facility in Italy. The RDIF on Monday said it has also made "agreements with companies from Italy, Spain, France and Germany to launch production of Sputnik V."
Do the agreements mean Sputnik’s launch across the EU is imminent?
Not exactly.
Both RDIF head Kirill Dmitriev and Adienne head Antonio Di Naro have stressed that the production agreement would guarantee a steady supply of the vaccine to Europe only once it’s approved by the EMA. Similarly, it is expected that other member states that have concluded production agreements for Sputnik V would produce it only after receiving the green light from the EMA.
Meanwhile, Reuters has reported that the EU would be willing to start purchasing negotiations with Russia after at least four of its members request the talks. Italy is reportedly lobbying fellow EU leaders to consider Sputnik V in an effort to vaccinate more people, Reuters reported.
But it remains unclear whether production or purchase agreements would influence the speed or outcome of the EMA’s eventual decision to approve Sputnik V for use across the 27-member bloc.
Will Russia be able to supply enough vaccines to Europe?
Questions have been raised over Russia’s ability to supply enough doses of Sputnik V to Europe once the vaccine is authorized. Last month, European Commission president Ursula von der Leyen criticized Russian offers to supply Europe with vaccines while Russia’s domestic vaccination program is stuttering.
“We still wonder why Russia is offering, theoretically, millions and millions of doses while not sufficiently progressing in vaccinating its own people,” she said.
Russia’s initial production issues were further underlined when the country under-delivered on vaccine supplies to Hungary, which received only a third of the 300,000 doses it had been expecting in January.
Vitaly Shakhnazarov, quality director at the COREX pharmaceutical logistics firm which works in Russia and eastern Europe, told The Moscow Times that he believes the production of Sputnik V has since been streamlined.
“Export to Europe is now becoming a very realistic option,” he said, but added that a majority of the Sputnik V doses will need to be produced within the EU in order to speed up the vaccine distribution process.
The FT has previously reported that Sputnik V faced global production hurdles in countries like India and South Korea.
Why is Sputnik V controversial in Europe?
Russia’s announcement in August that it had authorized the world’s first coronavirus vaccine ahead of its final clinical trials sparked skepticism from experts. International observers also raised questions over the lack of transparency with Russia’s early vaccine data.
Peer-reviewed research published in The Lancet medical journal last month showed Sputnik V to be 91.6% effective, easing some of the concerns surrounding the vaccine.
Western officials have also called Russia’s global vaccine supply efforts a Kremlin propaganda strategy, as Russia has touted Sputnik V as cheaper and easier to store than its western competitors.
AFP contributed reporting.
A Message from The Moscow Times:
Dear readers,
We are facing unprecedented challenges. Russia's Prosecutor General's Office has designated The Moscow Times as an "undesirable" organization, criminalizing our work and putting our staff at risk of prosecution. This follows our earlier unjust labeling as a "foreign agent."
These actions are direct attempts to silence independent journalism in Russia. The authorities claim our work "discredits the decisions of the Russian leadership." We see things differently: we strive to provide accurate, unbiased reporting on Russia.
We, the journalists of The Moscow Times, refuse to be silenced. But to continue our work, we need your help.
Your support, no matter how small, makes a world of difference. If you can, please support us monthly starting from just $2. It's quick to set up, and every contribution makes a significant impact.
By supporting The Moscow Times, you're defending open, independent journalism in the face of repression. Thank you for standing with us.
Remind me later.