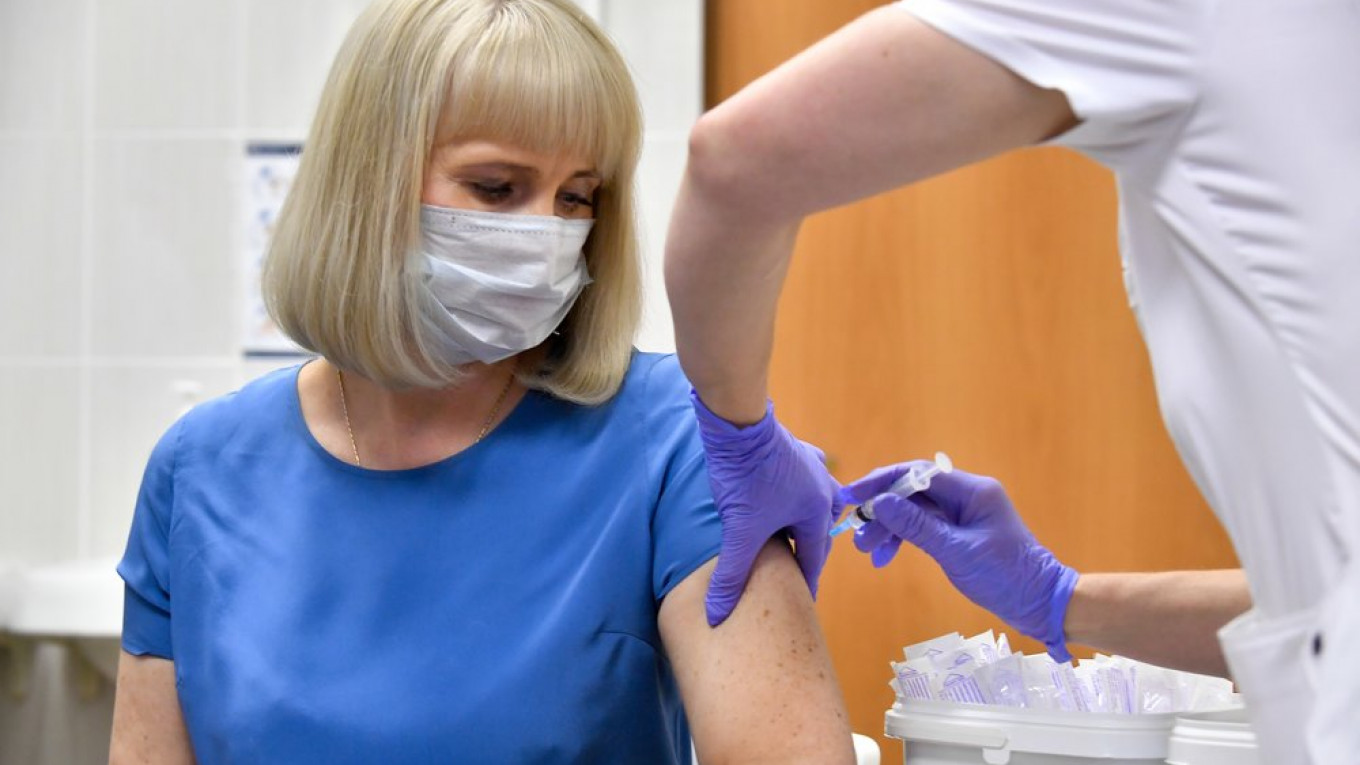
Russia has cleared two antiviral drugs called remdesivir for coronavirus treatment after doctors used the drugs to treat U.S. President Donald Trump, the Health Ministry announced Wednesday.
U.S. studies have said that biochemical firm Gilead Sciences’ remdesivir can shorten coronavirus recovery time by an average of four days but does not reduce deaths. Remdesivir was part of a “kitchen sink” of therapeutics administered to Trump after he was hospitalized with Covid-19 earlier this month.
“The Russian Health Ministry has registered two remdesivir antiviral drugs for the treatment of the new coronavirus infection (Covid-19),” it said in a statement.
“Registration licenses for domestic and imported drugs were issued Wednesday,” the Russian ministry added.
Remdesivir has received full approval in Japan and emergency use approval from the U.S. Food and Drug Administration, India and South Korea. Europe’s healthcare regulator recommended conditional approval for remdesivir this summer.
Remdesivir, which was initially intended as an Ebola drug, is estimated to cost more than $5,000 in the U.S. and less than $100 in India.
The drug is currently administered through an IV, with research of an inhaler version currently underway.
Russia is the world’s fourth-most affected country with more than 1.35 million confirmed cases of Covid-19.
The country is currently experiencing a second coronavirus wave with new cases nearly tripling from around 5,000 to nearly 14,000 in the course of a month. It has registered two candidate coronavirus vaccines ahead of large-scale clinical trials, with a third potential vaccine expected to be approved in December.
A Message from The Moscow Times:
Dear readers,
We are facing unprecedented challenges. Russia's Prosecutor General's Office has designated The Moscow Times as an "undesirable" organization, criminalizing our work and putting our staff at risk of prosecution. This follows our earlier unjust labeling as a "foreign agent."
These actions are direct attempts to silence independent journalism in Russia. The authorities claim our work "discredits the decisions of the Russian leadership." We see things differently: we strive to provide accurate, unbiased reporting on Russia.
We, the journalists of The Moscow Times, refuse to be silenced. But to continue our work, we need your help.
Your support, no matter how small, makes a world of difference. If you can, please support us monthly starting from just $2. It's quick to set up, and every contribution makes a significant impact.
By supporting The Moscow Times, you're defending open, independent journalism in the face of repression. Thank you for standing with us.
Remind me later.